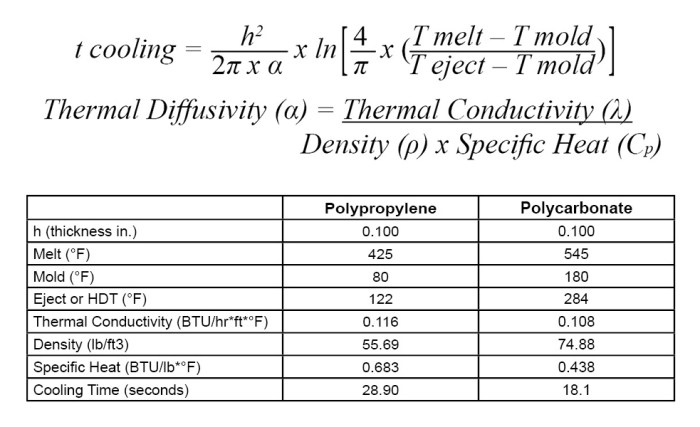
Density of benzene in lb/ft3 – Unveiling the Density of Benzene: Embark on an enlightening journey into the realm of benzene’s density, measured in lb/ft3. This physical property holds profound significance in characterizing benzene and unraveling its industrial applications.
Delving deeper, we’ll explore the factors that influence benzene’s density, including temperature and pressure. Moreover, we’ll uncover how this crucial property finds practical applications in determining the purity of benzene and optimizing industrial processes. Brace yourself for a captivating exploration of benzene density!
Physical Properties of Benzene

Benzene, a hydrocarbon compound, is renowned for its unique properties that have made it a valuable industrial solvent and a precursor for various chemical syntheses. Let’s delve into the physical characteristics of benzene, exploring its molecular structure, physical state, and other notable properties.
Chemical Formula and Molecular Structure
Benzene is represented by the chemical formula C6H6, indicating its composition of six carbon atoms and six hydrogen atoms. Its molecular structure is characterized by a hexagonal ring of carbon atoms with alternating single and double bonds, resulting in a planar and symmetrical configuration.
Physical State, Color, and Odor
At room temperature, benzene exists as a colorless liquid with a distinctive aromatic odor. It is highly volatile and evaporates readily, contributing to its strong smell. Benzene’s liquid form exhibits a low viscosity, allowing it to flow easily.
Significance of Low Viscosity and High Volatility
Benzene’s low viscosity and high volatility are crucial properties that contribute to its usefulness as a solvent. Its low viscosity enables it to penetrate and dissolve various substances, while its high volatility allows for easy evaporation, facilitating the removal of benzene from the dissolved mixture.
Density of Benzene
Density, a crucial material characteristic, quantifies the mass per unit volume. It plays a vital role in various industrial applications, including fluid dynamics, buoyancy calculations, and material selection.
Density of Benzene in lb/ft3
The density of benzene, a widely used aromatic hydrocarbon, is 55.44 lb/ft 3(889.2 kg/m 3). This value indicates that benzene is denser than water (62.43 lb/ft 3) but less dense than gasoline (42-48 lb/ft 3).
The high density of benzene contributes to its suitability as a solvent and fuel additive. Its relatively low volatility and high flash point make it a safer option compared to other volatile organic compounds (VOCs).
The density of benzene in lb/ft3 is a crucial parameter in various engineering applications. Measuring acme threads, a type of screw thread with a trapezoidal cross-section, is also essential in mechanical engineering. To accurately measure acme threads, a comprehensive guide is available here . Understanding the density of benzene in lb/ft3 and the techniques for measuring acme threads enables engineers to design and optimize systems involving these parameters.
Comparison to Other Liquids
The table below compares the density of benzene to other common liquids:
| Liquid | Density (lb/ft3) |
|---|---|
| Water | 62.43 |
| Benzene | 55.44 |
| Gasoline | 42-48 |
Factors Affecting Benzene Density: Density Of Benzene In Lb/ft3
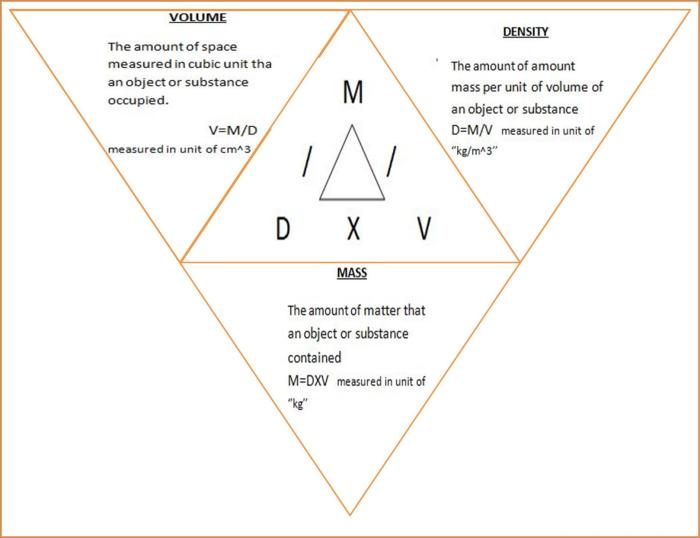
The density of benzene is influenced by various factors, primarily temperature and pressure. Understanding these factors is crucial for accurate measurements and calculations involving benzene.
Temperature
Temperature significantly affects the density of benzene. As temperature increases, the kinetic energy of benzene molecules increases, causing them to move faster and occupy more space. Consequently, the density of benzene decreases with increasing temperature.
Pressure
Pressure also plays a role in determining the density of benzene. When pressure is applied to benzene, the molecules are forced closer together, resulting in a higher density. However, this effect is relatively small compared to the impact of temperature.
Density of Benzene at Different Temperatures and Pressures, Density of benzene in lb/ft3
The following table provides the density of benzene at various temperatures and pressures:
| Temperature (°C) | Pressure (atm) | Density (lb/ft3) |
|---|---|---|
| 0 | 1 | 55.12 |
| 20 | 1 | 54.69 |
| 40 | 1 | 54.27 |
| 60 | 1 | 53.86 |
| 80 | 1 | 53.45 |
| 100 | 1 | 53.05 |
| 120 | 1 | 52.66 |
Applications of Benzene Density

The density of benzene is a crucial property that finds diverse applications across various fields. It serves as a key parameter in determining the purity of the compound, optimizing industrial processes, and addressing environmental concerns.
Purity Determination
Benzene density plays a significant role in assessing the purity of benzene. Pure benzene has a specific density at a given temperature. Any deviation from this value indicates the presence of impurities, such as other hydrocarbons or water. By measuring the density of a benzene sample, one can estimate its purity and identify potential contaminants.
Industrial Process Optimization
Benzene density is a critical factor in designing and optimizing industrial processes involving benzene. For instance, in the petrochemical industry, the density of benzene is used to determine the appropriate storage and transportation conditions. It also helps optimize distillation and separation processes by controlling the temperature and pressure parameters.
Environmental Science
Benzene density is relevant in environmental science, particularly in assessing the impact of benzene spills or leaks. By measuring the density of benzene in soil or water samples, environmental scientists can estimate the extent of contamination and develop appropriate remediation strategies.
FAQ Resource
What is the significance of benzene density?
Benzene density is a crucial parameter for characterizing the physical properties of benzene and understanding its behavior in various applications.
How does temperature affect benzene density?
Temperature inversely affects benzene density. As temperature increases, benzene density decreases due to the expansion of the liquid.
What are the industrial applications of benzene density?
Benzene density finds applications in determining the purity of benzene, designing and optimizing industrial processes, and in fields such as chemical engineering and environmental science.