Which of the following molecules contains a nonpolar covalent bond – In the realm of chemistry, the concept of nonpolar covalent bonds holds great significance. Nonpolar covalent bonds are formed when atoms share electrons equally, resulting in a neutral distribution of charge. Understanding which molecules contain nonpolar covalent bonds is crucial for comprehending their properties and behavior.
This exploration delves into the intricacies of nonpolar covalent bonds, examining the role of electronegativity, the properties of molecules with such bonds, and their practical applications. By unraveling the nature of these bonds, we gain insights into the fundamental building blocks of matter.
Nonpolar Covalent Bond
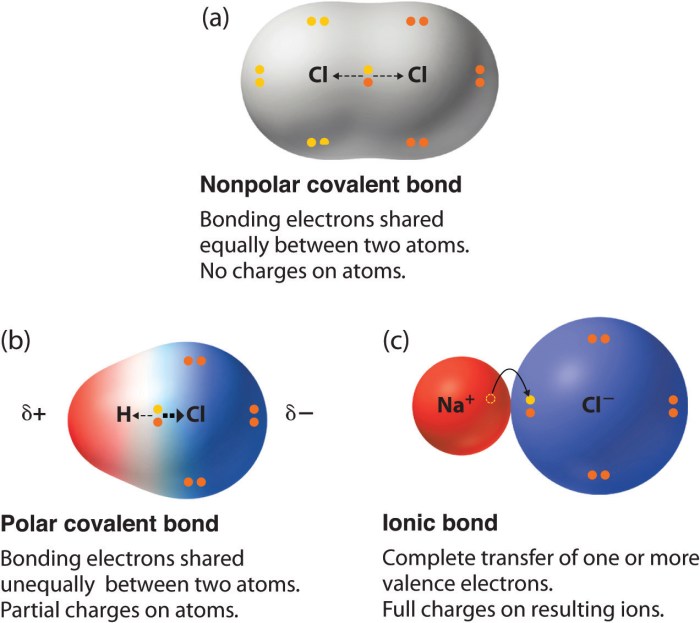
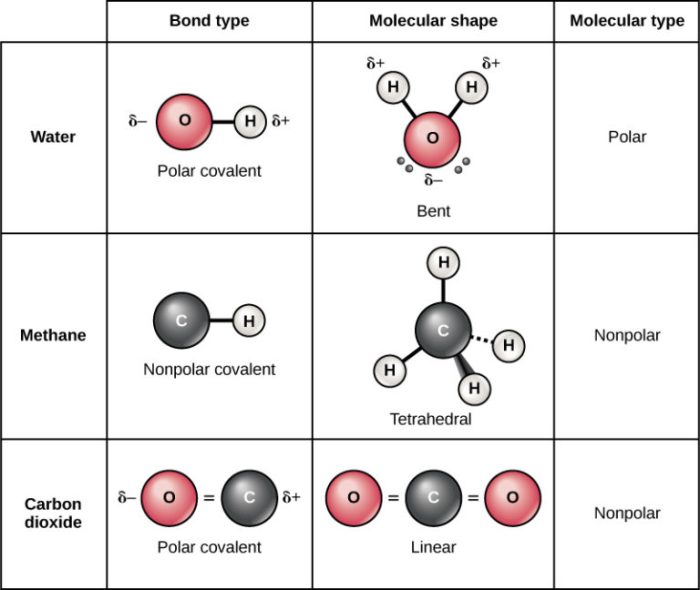
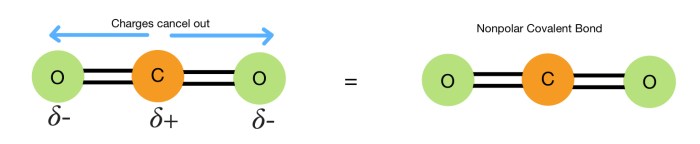
A nonpolar covalent bond is a chemical bond between two atoms where the electrons are shared equally between the atoms. This means that the electronegativity of the two atoms is the same or very similar. Nonpolar covalent bonds are typically found in molecules that are composed of the same type of atom, such as H2, O2, and N2.
Electronegativity and Nonpolar Covalent Bonds, Which of the following molecules contains a nonpolar covalent bond
Electronegativity is a measure of the ability of an atom to attract electrons. The greater the electronegativity of an atom, the more strongly it attracts electrons. In a nonpolar covalent bond, the electronegativity of the two atoms is the same or very similar.
This means that the electrons are shared equally between the atoms, and there is no net dipole moment.
Properties of Molecules with Nonpolar Covalent Bonds
Molecules with nonpolar covalent bonds are typically nonpolar. This means that they do not have a net dipole moment. Nonpolar molecules are attracted to each other by van der Waals forces. Van der Waals forces are weak intermolecular forces that are caused by the temporary fluctuations in the electron distribution of a molecule.
Examples of Molecules with Nonpolar Covalent Bonds
Some examples of molecules with nonpolar covalent bonds include:
- H2
- O2
- N2
- CH4
- CCl4
Applications of Nonpolar Covalent Bonds
Nonpolar covalent bonds are found in a wide variety of materials, including plastics, fuels, and lubricants. Nonpolar materials are often used in applications where it is important to have a material that is resistant to water and other polar solvents.
FAQ: Which Of The Following Molecules Contains A Nonpolar Covalent Bond
What is a nonpolar covalent bond?
A nonpolar covalent bond is formed when two atoms share electrons equally, resulting in a neutral distribution of charge.
How does electronegativity affect the polarity of a bond?
Electronegativity is a measure of an atom’s ability to attract electrons. The greater the difference in electronegativity between two atoms, the more polar the bond between them.
What are the properties of molecules with nonpolar covalent bonds?
Molecules with nonpolar covalent bonds are typically gases or liquids at room temperature. They are generally nonpolar and do not dissolve in water.