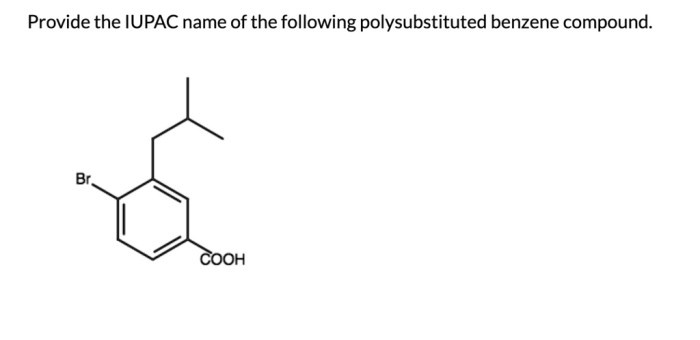
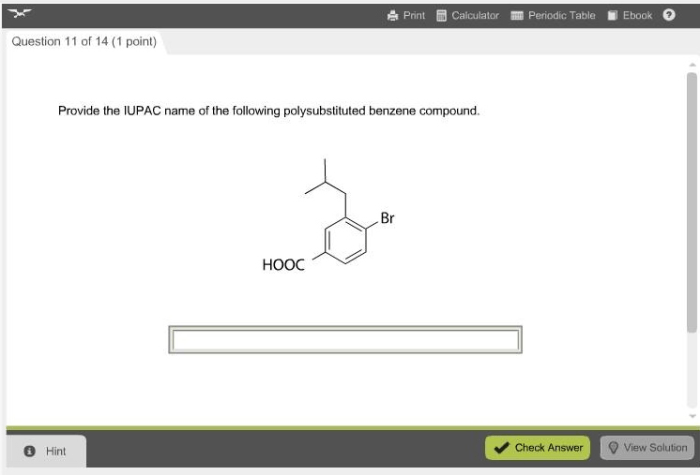
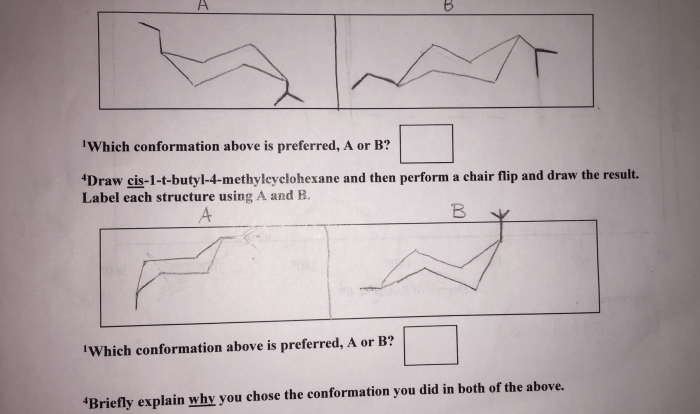
Provide the IUPAC name of the following polysubstituted benzene compound. This task requires a deep understanding of the rules and principles governing IUPAC nomenclature for polysubstituted benzenes. By following a systematic approach, we can accurately assign the correct IUPAC name to the given compound.
The International Union of Pure and Applied Chemistry (IUPAC) has established a set of guidelines for naming organic compounds, including polysubstituted benzenes. These guidelines ensure consistency and clarity in chemical nomenclature, facilitating effective communication among chemists.
IUPAC Nomenclature of Polysubstituted Benzenes
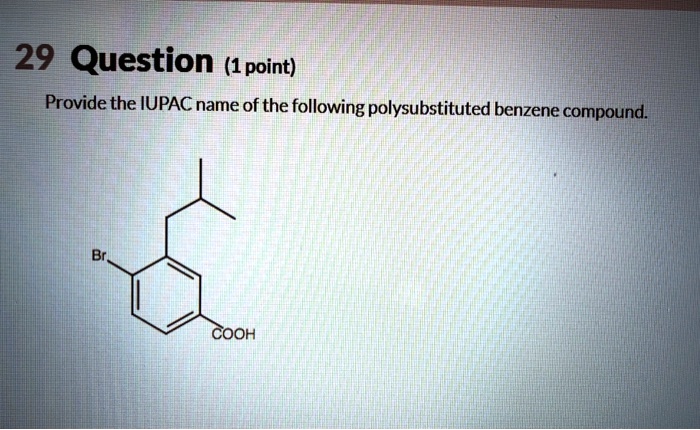
The International Union of Pure and Applied Chemistry (IUPAC) has established a set of rules for naming polysubstituted benzenes. These rules ensure that the names of these compounds are unique and unambiguous.
The basic rules for naming polysubstituted benzenes are as follows:
- The name of the parent compound is benzene.
- The substituents are named in alphabetical order.
- The number of each substituent is indicated by a number.
- The name of the compound is written as one word.
For example, the IUPAC name of the compound shown below is 1,2-dichlorobenzene.
Cl Cl | | C6H4
Identifying Substituents, Provide the iupac name of the following polysubstituted benzene compound
The first step in naming a polysubstituted benzene is to identify the substituents. Substituents can be any atom or group of atoms that is attached to the benzene ring.
The most common substituents are:
- Halogens (F, Cl, Br, I)
- Alkyl groups (CH3, C2H5, C3H7, etc.)
- Aryl groups (C6H5, C6H4CH3, etc.)
- Hydroxy groups (OH)
- Amino groups (NH2)
- Nitro groups (NO2)
When identifying substituents, it is important to remember that the benzene ring is a flat, hexagonal molecule. This means that the substituents can be attached to any of the six carbon atoms in the ring.
Numbering the Benzene Ring
Once the substituents have been identified, the next step is to number the benzene ring. The benzene ring is numbered in such a way that the substituents have the lowest possible numbers.
The rules for numbering the benzene ring are as follows:
- The carbon atom with the highest number of substituents is assigned the number 1.
- The other carbon atoms are numbered in a clockwise direction.
- If two or more substituents have the same number, the substituent that is attached to the carbon atom with the lowest number is assigned the lower number.
For example, the benzene ring shown below is numbered as follows:
1 2 | | 3 4 | | 5 6
Writing the IUPAC Name
Once the benzene ring has been numbered, the next step is to write the IUPAC name of the compound. The IUPAC name of a polysubstituted benzene is written as follows:
- The name of the parent compound is benzene.
- The names of the substituents are listed in alphabetical order, followed by the number of each substituent.
- The name of the compound is written as one word.
For example, the IUPAC name of the compound shown below is 1,2-dichlorobenzene.
Cl Cl | | C6H4
Special Cases
There are a few special cases that must be considered when naming polysubstituted benzenes.
One special case is when the benzene ring is substituted with two or more identical substituents. In this case, the prefix “di-“, “tri-“, “tetra-“, etc. is used to indicate the number of substituents.
For example, the IUPAC name of the compound shown below is 1,2,3-trichlorobenzene.
Cl Cl Cl | | | C6H3
Another special case is when the benzene ring is substituted with two or more different substituents that have the same priority. In this case, the substituents are listed in alphabetical order, followed by the number of each substituent.
For example, the IUPAC name of the compound shown below is 1-bromo-2-chlorobenzene.
Br Cl | | C6H4
Quick FAQs: Provide The Iupac Name Of The Following Polysubstituted Benzene Compound
What are the key rules for naming polysubstituted benzenes using IUPAC nomenclature?
The key rules include identifying the principal substituent, numbering the benzene ring to give the principal substituent the lowest possible number, and using prefixes to indicate the number and position of other substituents.
How do you determine the principal substituent in a polysubstituted benzene?
The principal substituent is the one with the highest priority according to the IUPAC priority rules. Priority is based on atomic number, degree of unsaturation, and other factors.
What are some examples of polysubstituted benzenes and their IUPAC names?
Examples include 1,2-dichlorobenzene, 1,3,5-trimethylbenzene, and 2-bromo-4-nitrophenol.